Have you ever wondered how long pharmaceutical companies can exclusively sell their drugs? The answer lies in the length of their drug patents. Patents are a crucial aspect of the pharmaceutical industry, and understanding how they work is essential to understanding the industry’s inner workings.
Drug patents can last for many years, allowing pharmaceutical companies to retain exclusive rights to manufacture and sell their drugs. However, the length of these patents can vary, and understanding the factors that influence patent length is crucial for both the pharmaceutical industry and consumers. In this article, we will explore the intricacies of drug patents and their impact on the industry.
How Long Do Drug Patents Last?
Pharmaceutical companies invest millions of dollars in developing new drugs. They also need to recoup their investment and make a profit. One way they do this is by obtaining a patent for their drug, which gives them exclusive rights to manufacture and sell it for a certain period of time. But how long do drug patents last? Let’s find out.
Patent Duration
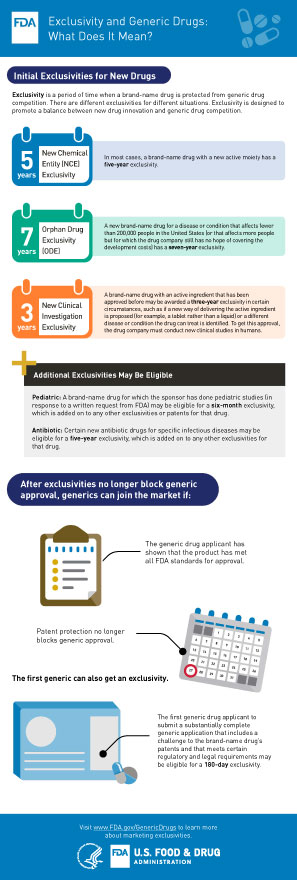
Each country has its own laws governing patents, including the duration of protection. In the United States, drug patents typically last for 20 years from the date of filing. However, the actual period of exclusivity can be shorter due to various factors, such as the time it takes to obtain FDA approval.
It’s worth noting that the 20-year duration applies to the patent as a whole, not just the drug itself. This means that a company may obtain multiple patents for a single drug, covering different aspects such as its formulation or method of use. In such cases, the overall period of exclusivity may be longer than 20 years.
Extensions and Generics
Even after a drug patent expires, the company that developed it may still enjoy some degree of exclusivity. For example, they may have obtained additional patents covering improvements or new uses of the drug. Alternatively, they may have secured a period of market exclusivity through regulatory mechanisms such as data exclusivity or orphan drug status.
Once all forms of exclusivity have expired, other companies are free to manufacture and sell generic versions of the drug. Generics are identical to the original drug in terms of active ingredients, dosage form, and route of administration. However, they may have minor differences in inactive ingredients or appearance due to different manufacturing processes.
Benefits of Drug Patents
Drug patents serve several important purposes. They incentivize pharmaceutical companies to invest in research and development, which can lead to the discovery of new drugs that improve health outcomes and quality of life. They also protect the intellectual property of the company, which can encourage innovation and prevent copying by competitors.
Furthermore, drug patents can provide a source of revenue for the company, which can be reinvested in further research or used to fund other activities. This, in turn, can benefit society as a whole by promoting economic growth and job creation.
Challenges of Drug Patents
Despite their benefits, drug patents also face several challenges. One of the most significant is the high cost of drugs, which can limit access to essential medicines for some patients. This is particularly true in developing countries, where the prices of patented drugs may be unaffordable for many people.
Other challenges include the potential for abuse of the patent system, such as by obtaining patents for minor variations of existing drugs or using legal loopholes to extend exclusivity. These practices can delay the availability of cheaper generic drugs and increase healthcare costs.
Patent vs. Generic Drugs
When a drug goes off-patent, generic versions become available at a lower cost. This can be beneficial for patients who may not have been able to afford the branded drug. However, there are also some differences between branded and generic drugs that patients should be aware of.
For example, the inactive ingredients in a generic drug may be different from those in the branded version. This can lead to differences in how the drug is absorbed and metabolized by the body. In some cases, this may result in differences in efficacy or side effects.
Conclusion
Drug patents are an important part of the pharmaceutical industry, providing incentives for innovation and protecting intellectual property. However, they also face challenges such as high costs and potential abuse of the system. Patients should be aware of the differences between branded and generic drugs, and consult with their healthcare provider to determine the best option for their specific needs.
| Patent Duration: | 20 years from the date of filing |
|---|---|
| Extensions and Generics: | Additional patents or regulatory mechanisms may provide some degree of exclusivity. Once all forms of exclusivity have expired, other companies can manufacture and sell generic versions of the drug. |
| Benefits of Drug Patents: | Incentivize research and development, protect intellectual property, and provide a source of revenue for the company. |
| Challenges of Drug Patents: | High costs and potential abuse of the system can limit access to essential medicines and delay the availability of cheaper generic drugs. |
| Patent vs. Generic Drugs: | Generic drugs are typically identical to branded drugs in terms of active ingredients, but may have minor differences in inactive ingredients that can affect efficacy and side effects. |
Frequently Asked Questions
Here are some common questions related to drug patents and their duration.
What is a drug patent?
A drug patent is a legal document granted by a government agency that gives the patent holder exclusive rights to produce and sell a particular drug for a certain period of time. During this time, no other company can produce or sell the same drug without permission from the patent holder.
In the United States, drug patents are issued by the U.S. Patent and Trademark Office (USPTO). The purpose of a drug patent is to give the patent holder an opportunity to recoup the costs of developing and testing a new drug, as well as to make a profit from its sale.
What is the duration of a drug patent?
The duration of a drug patent can vary depending on the country where the patent is granted. In the United States, the duration of a drug patent is generally 20 years from the date of filing. However, there are some exceptions to this rule.
For example, if a drug is granted FDA approval after the patent has been filed, the patent holder may be able to extend the duration of the patent by up to five years. Additionally, if the drug is granted orphan drug status, the patent holder may be able to extend the duration of the patent by up to seven years.
What happens when a drug patent expires?
When a drug patent expires, other companies are free to produce and sell generic versions of the drug. Generic drugs are equivalent to the original drug in terms of safety, quality, and effectiveness, but they are typically sold at a lower price because the manufacturer does not have to recoup the costs of developing the drug.
This competition can lead to lower prices for consumers and can increase access to the drug in question. However, it can also lead to a decrease in revenue for the original patent holder.
Can drug patents be challenged?
Yes, drug patents can be challenged in court. One common way to challenge a drug patent is to argue that the patent is invalid because the drug in question is not sufficiently innovative or because the patent holder did not meet the requirements for obtaining a patent.
Another way to challenge a drug patent is to argue that the patent holder engaged in anticompetitive behavior, such as by filing frivolous lawsuits against generic manufacturers or by making false or misleading statements to the USPTO. If a court finds that a drug patent is invalid or unenforceable, other companies may be able to produce and sell generic versions of the drug.
What are the benefits of drug patents?
Drug patents can provide several benefits. First, they encourage innovation by giving companies an opportunity to recoup the costs of developing and testing new drugs. This can lead to the development of new and better treatments for a wide range of medical conditions.
Second, drug patents can help to ensure that drugs are safe and effective by requiring companies to conduct extensive testing before bringing a drug to market. Finally, drug patents can help to maintain a stable and predictable regulatory environment, which can be beneficial for both companies and consumers.
How Long Do Drug Patents Last DrugPatentWatch
In conclusion, drug patent laws have a significant impact on the pharmaceutical industry. Patents allow companies to protect their investment in research and development, but also limit competition and increase drug prices. The length of a drug patent varies from country to country, but in general, patents last for 20 years from the date of filing.
It’s important to note that the patent clock starts ticking before a drug is even approved by regulatory agencies, leaving companies with less time to recoup their costs. However, there are ways for companies to extend their patent protection, such as by obtaining additional patents for new uses or formulations of the same drug.
Overall, the length of drug patents is a complex issue with pros and cons for both pharmaceutical companies and consumers. As the industry continues to evolve, it will be interesting to see how patent laws adapt to balance the need for innovation and affordability in healthcare.